Principle:
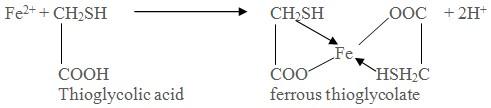
Limit test of Iron is based on the reaction of iron in ammonical solution with thioglycollic acid in presence of citric acid to form iron thioglycolate which is pale pink to deep reddish purple in color.
Procedure:
|
Test sample
|
Standard compound
|
|
Sample is dissolved in specific amount of water and then volume is made up to 40 ml
|
2 ml of standard solution of iron diluted with water upto 40ml
|
|
Add 2 ml of 20 % w/v of citric acid (iron free)
|
Add 2 ml of 20 % w/v of citric acid (iron free)
|
|
Add 2 drops of thioglycollic acid
|
Add 2 drops of thioglycollic acid
|
|
Add ammonia to make the solution alkaline and adjust the volume to 50 ml
|
Add ammonia to make the solution alkaline and adjust the volume to 50 ml
|
|
Keep aside for 5 min
|
Keep aside for 5 min
|
|
Color developed is viewed vertically and compared with standard solution
|
Color developed is viewed vertically and compared with standard solution
|
Earlier aamonium thiocyanate reagent was used for the limit test of iron. Since thioglycolic acid is more sensitive reagent, it has replaced ammonium thiocyanate in the test.
Observation:
The purple color produce in sample solution should not be greater than standard solution. If purple color produces in sample solution is less than the standard solution, the sample will pass the limit test of iron and vice versa.
Reasons:
Citric acid helps precipitation of iron by ammonia by forming a complex with it.
Thioglycolic acid helps to oxidize iron (II) to iron (III).
Ammonia to make solution alkaline