The electric charge is given by:
Q = I ∙ t
Corresponding SI units:
coulomb (C) = ampere (A) ∙ second (s)
Where I is the electric current and t is the time (duration).
- Electric charge is a fundamental property like mass, length etc associated with elementary particles for example electron, proton and many more.
- Electric charge is the property responsible for electric forces which acts between nucleus and electron to bind the atom together.
- Charges are of two kinds
(i) negative charge
(ii) positive charge
- Electrons are negatively charged particles and protons, of which nucleus is made of, are positively charged particles. Actually nucleus is made of protons and neutrons but neutrons are uncharged particles.
- Electric force between two electrons is same as electric force between two protons kept at same distance apart i. e., both set repel each other but electric force between an electron and proton placed at same distance apart is not repulsive but attractive in nature.
(a) Like charges repel each other
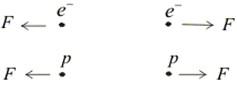
(b) Unlike charges attract each other

- Assignment of negative charge on electron and positive charge on proton is purely conventional; it does not mean that charge on electron is less than that on proton.
- Importance of electric forces is that it encompasses almost each and every field associated with our life; being it matter made up of atoms or molecules in which electric charges are exactly balanced or adhesive forces of glue associated with surface tension, all are electric in nature.
Unit
- Charge on a system can be measured by comparing it with the charge on a standard body.
- SI unit of charge is Coulomb written as C.
- 1 Coulomb is the charge flowing through the wire in 1 second if the electric current in it is 1A.
- Charge on electron is -1.602 * 10 -19 C and charge on proton is positive of this value.
- Two important properties of charge are Quantization and Conservation.
(a) Quantization of charge
(i) Electric charge can exist only as an integral multiple of charge on an electron (-e) i.e.
q = ± ne, where n is an integer.
(ii) The Possible values of electric charge are q = ± 1e; ± 2e; ± 3e...
(iii) Charge less than the charge on an electron (i.e. e = 1.6 * 10-19 C) is not possible.
(b) Conservation of charge
(i) On an isolated system, total electric charge always remains constant.
(ii) Total charge on a body is equal to the algebraic sum of all the charges present on it. Every atom is electrically neutral as it contains as many electrons as the number of protons in it.
(iii) When we rub a glass rod with a piece of silk, the positive charge acquired by the glass rod is equal to negative charge acquired by the silk piece. Thus charges are produced in equal and unlike pairs.
Example (1): What is the possible value of electric charge?
(a) 1 X 1.6 X 10-19 C
(b) 2.4 X 1.6 X 10-19 C
(c) -8 X 1.6 X 10-19 C
(d) 1 X 1.8 X 10-19 C
Solution: (a)
As we know that, electric charge can exist only as an integral multiple of charge on an electron (-e) i.e.
q = ±ne, where n is an integer. So q = ±1 X 1.6 X 10-19 C
Example (2): If n=2, what will be the value of electric charge? (Given e =1.6 X 10-19 C)
(a) ±0.8 X 10-19 C
(b) ±3.2 X 10-19 C
(c) ±4.3 X 10-19 C
(d) ±6.3 X 10-19 C
Solution: (b)
We know that
q = ±ne
= 2 X 1.6 X 10-19 C
= ±3.2 X 10-19 C
Hence option (b) is correct.
Example (3): Is charge less than the charge (i.e. e = 1.6 X 10-19 C) on an electron possible?
(a) Yes (b) No
Solution :( b) As we know
q = ±ne where n is an integer i.e. n= 1, 2, 3,...
Example 4): What is the total charge of all of the protons in 1.00 kg of carbon?
(a) 4.82 X 107 C
(b) 3.96 X 107 C
(c) 4.82 X 109 C
(d) 3.96 X 1012 C
Solution: (a) We can find the number of coulombs of positive charge there are in 1.00 kg of carbon from Q=6nce, where nc is the number of atoms in 1.00 kg of carbon and the factor of 6 is present to account for the presence of 6 protons in each atom. We can find the number of atoms in 1.00 kg of carbon by setting up a proportion relating Avogadro’s number NA , the mass of carbon, and the molecular mass of carbon to nc.